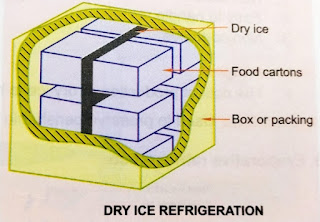
What is Dry ice refrigeration?
- In
this refrigeration method, solid carbon dioxide is used. Solid carbon dioxide is called
as Dry ice.
- It
has the characteristics of changing from solid to vapour state directly
without entering into the liquid phase. During this transfer, the dry ice
absorbs heat equivalent to its latent heat of evaporation.
- It
evaporates at -78.3°c at standard atmospheric pressure.
- The
dry ice is placed on top or at sides of cartons containing eatables, etc.
When this dry ice sublimates it will absorb heat from the
food stuff in the cartons or containers by keeping them in frozen
condition.
Production of dry ice
- The
solid carbon dioxide can be pressed into various sizes and shapes. while
absorbing the heat, it changes directly from solid to vapour and does not
go into liquid state. The figure shows the production of solid carbon
dioxide.
- The carbon dioxide vapour is compressed to high pressure and high-temperature vapours using a compressor. Then it is condensed to high-pressure liquid CO2 in the condenser. High-pressure liquid CO2 is made to pass through the expansion value.
- During
expansion, the pressure drops below triple point and a mixture of solid
and vapour CO2 is received in the snow chamber.
- The
solid CO2 in the form of snow is removed and compressed by mechanical
means to form CO2 cakes.
- The
vapour CO2 is mixed with make-up CO2 and once again the process repeats.
- During
the change of state from solid to vapour in the application areas, the dry
ice absorbs heat equivalent to its latent heat of evaporation.
Advantages
- Non-toxic.
- Easy
to handle.
- Non-corrosive.
Disadvantages
- The
cost of the production of dry ice is high.
Application
- Used
to preserve perishable food stuff during transportation.
- Used
to store medicines and vaccines.
Important terms to remember
Sublimation: It is a process by which a
substance changes from solid to vapour without entering liquid state.
Triple point: The only state at which the solid, liquid and vapour phases
coexist in equilibrium.
Cool facts about dry ice
- Charles
Thilorier is the first person to publish observations about dry ice in
1835.
- Compared
to water dry ice has heavier density and gravity.
- We
have seen the smoky effect at parties and concerts, these effects can be
done by dry ice.
Tags:
Refrigeration